PTEN
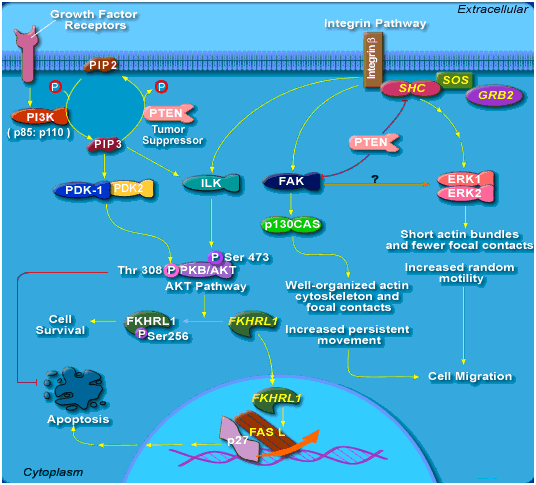
Phosphatase and tensin homolog (PTEN) is a protein that is encoded by the PTEN gene. Mutations of this gene are a step in the development of many cancers.PTEN acts as a tumor suppressor gene. When the PTEN enzyme is functioning properly, it acts as part of a chemical pathway that signals cells to stop dividing and can cause cells to undergo programmed cell death (apoptosis) when necessary.These functions prevent uncontrolled cell growth that can lead to the formation of tumors.There is also evidence that the protein made by the PTEN gene may play a role in cell movement (migration) and adhesion of cells to surrounding tissues. PTEN is one of the most commonly lost tumor suppressors in human cancer. During tumor development, mutations and deletions of PTEN occur that inactivate its enzymatic activity leading to increased cell proliferation and reduced cell death. Frequent genetic inactivation of PTEN occurs in glioblastoma, endometrial cancer, and prostate cancer; and reduced expression is found in many other tumor types such as lung and breast cancer.Up to 70 percent of men with prostate cancer have lost one copy of the PTEN gene at the time of diagnosis.PTEN mutation also causes a variety of inherited predispositions to cancer. Mutations in the PTEN gene cause several other disorders that, like Cowden syndrome, are characterized by the development of noncancerous tumors called hamartomas .
Although the intracellular functions of PTEN are complex and only partially deciphered, it appears that one of the most relevant consequences of the PTEN-defective phenotype is the activation of the PI3K/Akt signaling pathway.Akt mediates multiple intracellular functions pertaining to cell proliferation and apoptosis and has been implicated in chemoresistance in colon, bladder and ovarian cancers. Although the signaling pathways downstream from Akt are not totally elucidated, it is well established that mTOR is one of the most relevant mediators of Akt functions. mTOR is activated in response to Akt phosphorylation, resulting in the phosphorylation of p70S6 kinase and 4E-BP1, and, consequently, increases the translation of mRNAs of proteins involved in the regulation of the cell cycle. Inhibition of mTOR by rapamycin results in cell cycle arrest, p53-dependent and -independent apoptosis and tumor growth inhibition.
Many studies have now confirmed that resistance to anti-EGFR therapies is often linked to PTEN status in cancer cells, and PTEN loss negatively correlates with clinical response to these therapies or others.This problem can be overcome by the use of combined therapies which show synergistic effects and are now tested in clinical trials.MMPs overexpression is another resistance mechanism. We previously discussed the negative correlation between PTEN and MMPs expression; therefore, cancer cells with reduced or lost PTEN activity are more prone to develop this kind of resistance.To avoid the emergence of such resistance, different strategies aiming at antagonizing the PI3K pathway or reintroducing the expression and/or activity of PTEN have been tried. Many PI3K inhibitors, as well as Akt and mTOR inhibitors, are now being tested in clinical trials.Although there are numerous inhibitors of the PI3K pathway, to date no PTEN inducer has been found, explaining why only PTEN transfection or gene therapy has been tried.Transfection of wild-type PTEN into human prostate cancer cells sensitizes cells to radiation and leads to a decreased tumor-induced angiogenesis.
Loss of PTEN function from PTEN mutations was reported in 5%–10% of human breast cancers, and PTEN haploinsufficiency due to loss of heterozygosity at the PTEN locus can be found in nearly 50% of breast tumors. In addition, epigenetic downmodulation of PTEN has also been reported.
Recent findings show that PI3K inhibitors could overcome PTEN loss-induced trastuzumab resistance in ErbB2-overexpressing breast cancer cells in vitro and in vivo.This suggests that members of the PI3K pathway, as well as PTEN, are molecular targets for overcoming trastuzumab resistance.These new insights will guide the future development of agents for overcoming trastuzumab resistance and novel targeted cancer therapies to benefit cancer patients.