MET
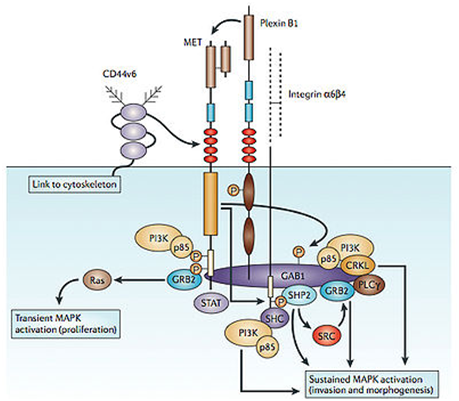
c-Met is a proto-oncogene that encodes a protein known as hepatocyte growth factor receptor (HGFR). Abnormal MET activation in cancer correlates with poor prognosis, where aberrantly active MET triggers tumor growth, formation of new blood vessels ( angiogenesis ) that supply the tumor with nutrients, and cancer spread to other organs ( metastasis ). MET is deregulated in many types of human malignancies, including cancers of kidney, liver, stomach, breast, and brain.
The MET signaling pathway is abnormal in a wide variety of cancers and stimulates cell growth, invasion, and metastasis, as well as promoting resistance to apoptosis. Because of its ubiquitous role in cancer cells, the MET axis has been seen as an attractive target for cancer therapy. Over the last four years, more than 10 anticancer agents targeting different aspects of MET signaling via different mechanisms have been introduced into the clinic. The majority of MET inhibitors are still in late phase I and phase II trials, but at least three compounds, tivantinib, onartuzumab, and cabozantinib, are in phase III trials in lung cancer and medullary thyroid cancer. It should also be noted that MET is expressed not only in tumor cells and endothelial cells, but also in osteoblasts (bone-forming cells) and osteoclasts (bone-removing cells). The MET pathway is abnormally regulated in a wide range of human cancers, including the most common epithelial cancers such as breast, colorectal, lung, pancreatic, hepatic, and ovarian cancers. MET inhibitors remain a very promising class of compounds for cancer therapy. While the preclinical data and knowledge of the biology of MET suggest that inhibiting MET may have a profound effect on cancer therapy, results from clinical trials to date suggest that MET inhibitors as single agents may be important only in a subset of patients. For instance, there were very few responses to foretinib in hereditary papillary RCC (which harbors c-MET mutations), and the few responses that were seen were not clearly connected to c-MET mutation status. This may imply that while many c-MET mutations have been described, a number of them may not be activating mutations that sensitize the tumors to MET inhibitors. Currently, the most robust single-agent activity in unselected patients has been seen with the multikinase inhibitors, such as cabozantinib.


